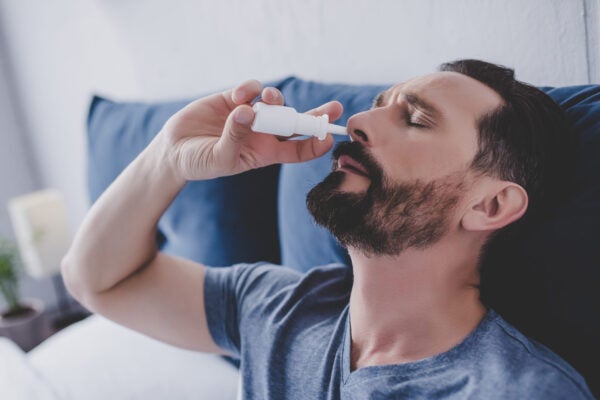
AUSTIN, Texas — A newly patented, testosterone-containing nasal spray developed by a psychology professor at The University of Texas at Austin could provide those suffering from testosterone deficiency and other ailments, such as anxiety disorders, with easily modulated, fast-acting results.
Currently, those diagnosed with “low T” — testosterone deficiency or hypogonadism — may receive hormone supplements via drops, transdermal creams and gels, injections and subcutaneous “seeds,” all of which take days and/or multiple dosages to reach full potential.
The new aqueous-based nasal spray was initially developed to address a current market need for comfortable and controlled dosages of testosterone for people suffering from decreased libido and anxiety disorders. Testosterone is in high demand with prescriptions increasing fivefold since 2011.
Although testosterone therapy is most often marketed and prescribed to men, UT Austin psychology professor Robert Josephs and MedCara Pharmaceuticals pharmacist Craig Herman developed their nasal spray in response to a long-standing research question about why women are twice as likely as men to develop anxiety disorders.
Research has shown that while there is no difference in anxiety disorders among prepubescents, puberty introduces a sharp uptick in anxiety disorders in girls, who naturally have about one-tenth the amount of testosterone as boys. The researchers speculated that men’s higher concentrations of circulating testosterone may protect against anxiety and began developing a treatment to address the issue.
“A growing body of research points to testosterone’s importance in the etiology of anxiety disorders. These findings highlight the potential benefit of rapid increases in testosterone concentration as a means to short-circuit the mechanisms underlying the development of anxiety-related disorders, including panic disorder, social anxiety and PTSD,” said Josephs, who is also an adjunct professor of psychiatry at the Dell Medical School.
Although testosterone is not currently prescribed for anxiety, Josephs hopes that treating anxiety disorders with a short-term, fast-acting testosterone product might be prescribed alongside a lower dose of benzodiazepines — such as Xanax or Klonopin.
“Although benzodiazepines work well, they have strong sedative effects,” Josephs said. “Testosterone is not sedating.”
The nasal spray (United States Patent No. 10,258,63, issued on April 16, 2019) has been licensed to Acerus Pharmaceuticals Corporation, which has the flexibility under the current agreement to use the technology in whatever medical field it deems most appropriate.
The University of Texas at Austin is committed to transparency and disclosure of all potential conflicts of interest. The university investigator who led this research, Robert Josephs, has submitted required financial disclosure forms with the university. Josephs holds intellectual property rights that may yield revenue from the discovery described in this research.