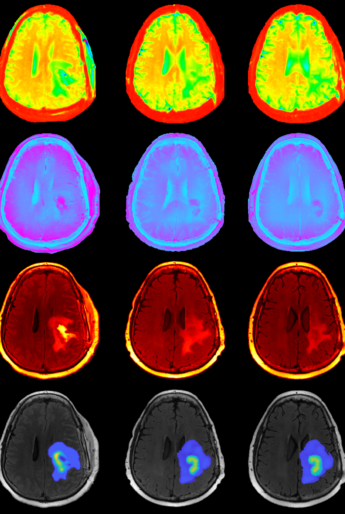
AUSTIN, Texas — A team studying malignant brain tumors has developed a new technique for predicting how individual patients will respond to chemoradiation, a major step forward in efforts to personalize cancer treatment.
Researchers at The University of Texas at Austin’s Oden Institute for Computational Engineering and Sciences, Texas Advanced Computing Center (TACC) and The University of Texas MD Anderson Cancer Center have merged various quantitative imaging measurements with computational simulations to create an accurate model for calculating the progression of high-grade glioma.
High-grade gliomas are the most common cancerous primary brain tumors found in adults. Current treatments involve surgical resection of the tumor followed by radiation therapy and chemotherapy. Despite this aggressive treatment, prognosis for patients who undergo this approach is generally poor. The growth and behavior of these tumors varies from patient to patient, making the need for techniques to personalize therapy on an individual patient level particularly important.
In a paper published in Nature Scientific Reports, the authors used a combination of anatomical and structural imaging to inform a computational mechanistic model that predicts for high-grade glioma tumor progression.
“This project couldn’t be attempted without close collaboration between engineers and clinicians,” said David Hormuth of the Center for Computational Oncology at UT Austin’s Oden Institute.
“Our approach of using individual patient imaging data in a predictive mechanistic model, that incorporates both the anatomical appearance of the tumor on MRI and measurements from a specific MRI scanning technique called diffusion tensor imaging, is showing real promise,” said Dr. Caroline Chung of MD Anderson.
Current radiation therapy methods are already tailored to patients using mostly anatomical imaging data prior to the start of radiation therapy and can be adapted in reaction to major changes in tumor appearance during treatment. However, this new technique is a first step toward providing radiation oncologists with the information they need to personalize treatment plans based on a predicted spatial map of the tumor’s resistance to radiation.
Throughout this project, researchers at the Oden Institute and MD Anderson have gone back and forth on the type of data needed, model components and the overall goal or application of this model. The Oden Institute brought the expertise in tumor mechanics and modeling, an innovative, physics-based research approach led by Tom Yankeelov of UT Austin over several years. Once paired with Chung’s quantitative imaging and clinical brain tumor expertise, the researchers successfully translated prior preclinical efforts in high-grade glioma.
TACC, the third partner in the collaboration to end cancer, made it possible for the researchers to simultaneously calibrate a large family of biologically based mathematical models for each patient.
“In total, we had roughly 6,000 different calibrations or forecast scenarios that would take years to run on a standard laptop,” Hormuth said. “By using the Lonestar 5 system to run our model calibration and forecasting approach in parallel, we were able to evaluate all of these scenarios in a matter of days.”