AUSTIN, Texas — By altering the genetic code in bacteria, researchers at The University of Texas at Austin have demonstrated a method to make therapeutic proteins more stable, an advance that would improve the drugs’ effectiveness and convenience, leading to smaller and less frequent doses of medicine, lower health care costs and fewer side effects for patients with cancer and other diseases.
The results were published today in the journal Nature Biotechnology.
Many drugs commonly used to treat cancer and diseases of the immune system — including insulin, human growth hormone, interferon and monoclonal antibodies — can have a short active life span in the human body. That’s because these drugs, which are proteins or chains of amino acids linked together by chemical bonds, contain the amino acid cysteine, which makes chemical bonds that break down in the presence of certain compounds found in human cells and blood.
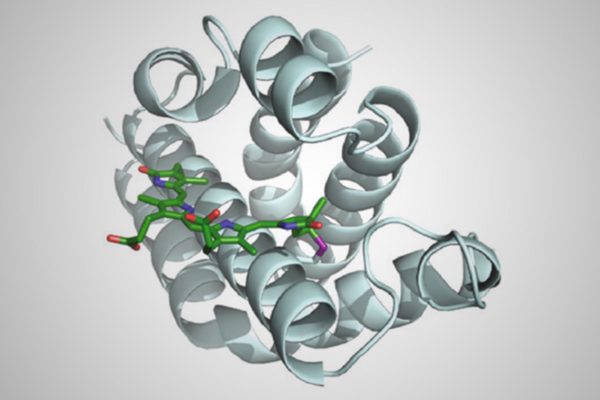
The new method replaces cysteine with another amino acid called selenocysteine, which forms hardier chemical bonds. The change would lead to drugs that have the same therapeutic benefit but increased stability and may survive longer in the body, according to the new study.
“We have been able to expand the genetic code to make new, biomedically relevant proteins,” said Andrew Ellington, associate director of the Center for Systems and Synthetic Biology and a professor of molecular biosciences who co-authored the study.
Biochemists have long used genetically modified bacteria as factories to produce therapeutic proteins. However, bacteria have built-in limitations that previously prevented harnessing selenocysteine in these therapies. Through a combination of genetic engineering and directed evolution — whereby bacteria that produce a novel protein containing selenocysteine can grow better than those that don’t — the researchers were able to reprogram a bacteria’s basic biology.
“We have adapted the bacteria’s natural process for inserting selenocysteine to remove all the limitations, allowing us to recode any position in any protein as a selenocysteine,” said Ross Thyer, a postdoctoral researcher in Ellington’s lab who led the study.
Other authors on the paper, all from UT Austin, are Raghav Shroff, Dustin Klein, Simon d’Oelsnitz, Victoria Cotham, Michelle Byrom and Jennifer Brodbelt.
Thyer, Brodbelt and Ellington described the basic method in a paper in the Journal of the American Chemical Society in 2015. In this latest study, the team demonstrated the practical application of this method by producing medically relevant proteins — including the functional region of the breast cancer drug Herceptin. The team showed that the new proteins survive longer in conditions similar to those found in the human body compared with existing proteins containing cysteine.
Funding for this research was provided by the Welch Foundation, the National Science Foundation, the U.S. Army Research Office and the National Cancer Institute.
The University of Texas at Austin is committed to transparency and disclosure of all potential conflicts of interest. University investigators involved in this research have submitted required financial disclosure forms with the university. UT Austin filed patent applications on the technology described in this news release, and the patents were licensed earlier this year to form a startup to develop improved protein therapeutics. Ellington and Thyer have equity ownership in the biotech startup.