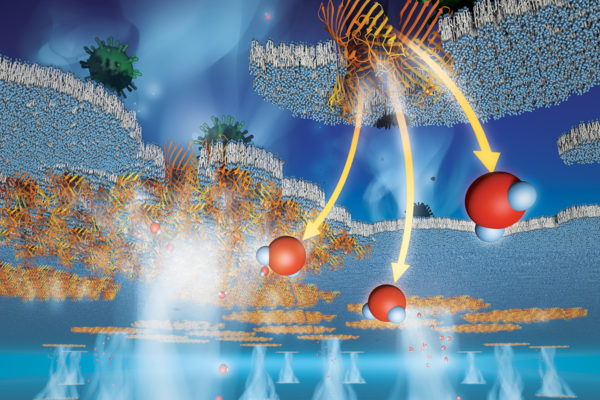
AUSTIN, Texas — A multidisciplinary team of engineers and scientists has developed a new class of filtration membranes for a variety of applications, from water purification to small-molecule separations to contaminant-removal processes, that are faster to produce and higher performing than current technology. This could reduce energy consumption, operational costs and production time in industrial separations.
Led by Manish Kumar, associate professor in the Cockrell School of Engineering at The University of Texas at Austin, the research team describes their new high-performance membranes in a recent issue of Nature Materials.
The team’s new filtration membranes demonstrate higher density of pores than that of commercial membranes and can be produced much faster — in two hours, versus the several-day process currently used. Until now, integrating protein-based membranes into current technology used for industrial separations has been challenging because of the amount of time needed to create these membranes and the low density of proteins in resulting membranes.
This comprehensive and collaborative research effort brought together engineers, physicists, biologists and chemists from UT Austin, Penn State University, University of Kentucky, University of Notre Dame and the company Applied Biomimetic. The work presents the first end-to-end synthesis of a true protein-based separation membrane with pores between half a nanometer and 1.5 nanometers in size. A nanometer is just a few times the size of a water molecule and a hundred thousand times smaller than the width of a human hair.
The membranes created by the team are biomimetic, meaning they mimic systems or elements of nature, and imitate those that naturally occur in cell membranes for transporting water and nutrients. They recently published another paper highlighting the inspiration for their method. High-density packing of these protein channels into polymer sheets forms protein pores within the membrane, similar to those seen in human eye lenses, but within a nonbiological polymer environment.
Three different biomimetic membranes were fabricated by the team and demonstrated a sharp, unique and tunable selectivity with three different pore sizes of membrane protein channels. The methods described can be adapted with the insertion of protein channels of different pore sizes or chemistries into polymer matrices to conduct specifically designed separations.
“In the past, attempts to make biomimetic membranes fell far short of the promise of these materials, demonstrating only two to three times improvement in productivity,” said Yu-Ming Tu, a UT Austin chemical engineering doctoral student and lead on the project. “Our work shows a surprising 20 to 1,000 times improvement in productivity over the commercial membranes. At the same time, we can achieve similar or better separation of small molecules, like sugars and amino acids, from larger molecules, like antibiotics, proteins and viruses.”
This high productivity was made possible by the very high density of pore proteins. Approximately 45 trillion proteins can fit onto the membrane, if it were the size of a U.S. quarter; the membranes created were 10-20 times larger in area. This pore density is 10 to 100 times higher than conventional filtration membranes with similar nano-sized pores. Additionally, all the pores in these membranes are exactly the same size and shape, allowing them to better retain molecules of desired sizes.
“This is the first time that the promise of biomimetic membranes involving membrane proteins has been translated from the molecular scale to high performance at the membrane scale,” Kumar said. “For so long, engineers and scientists have been trying to find solutions to problems only to find out nature has already done it and done it better. The next steps are to see if we can fabricate even larger membranes and to test whether they can be packaged into flat sheet and spiral-wound-type modules like the ones common in industry.”
The research was funded by the National Science Foundation.