AUSTIN, Texas — Research at The University of Texas at Austin’s PsyBrain Lab contributed to the recent Food and Drug Administration approval of the first commercially available laboratory test for traumatic brain injury (TBI) in the U.S. The technology, called Alinity i, tests for elevated levels of two biomarkers in blood that are tightly correlated with brain injury and can diagnose patients in as little as 18 minutes.
Prior to the FDA’s approval of Alinity i, which is produced by Abbott Laboratories, diagnosis of TBI relied on subjective assessment by doctors and CT scans to detect brain tissue damage or lesions. Many people who suspected they might have suffered a mild case of TBI, or a concussion, might not even see a doctor. The new diagnostic tool can provide greater certainty for patients and doctors and might encourage more people to seek medical attention for possible TBI.
“This work demonstrated the accuracy of a blood test that can reduce the number of unnecessary CT scans and reduce the amount of time a patient may spend in the emergency department,” said David Schnyer, professor and chair of the Department of Psychology who oversees PsyBrain Lab. “The Alinity i test can be used when a patient shows up to the hospital with a suspected TBI within 12 hours of injury.”
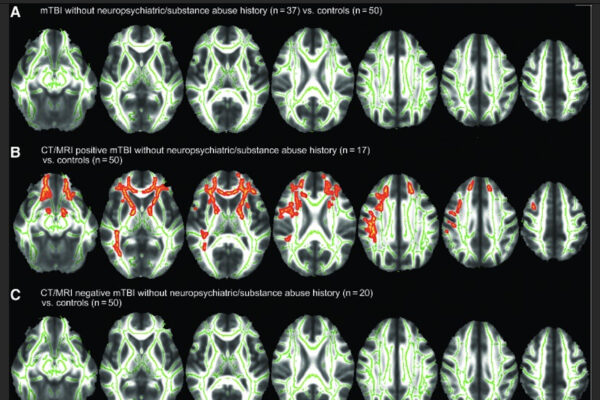
The research was part of the TRACK-TBI network, a multisite clinical trial network, and focused on diagnosis of mild TBI. PsyBrain Lab has been part of the network since its inception in 2008, helping both to identify the biomarkers that indicate TBIs and later to confirm the blood test’s accuracy in finding them. The tests showed it to be 99.4% accurate when reporting negative results. Patients who test negative are spared from more expensive and time-consuming testing techniques.
“It’s an advancement to celebrate,” said Robin McGee, chief study coordinator for PsyBrain Lab. “When educating patients, doctors and nurses ‘on the ground’ in the emergency department about our work, they are unequivocally impressed. They say, ‘A blood test for concussion?’”
Abbott Laboratories is next partnering in a study that will broaden the reach of the test to include evaluating TBI in children. Schnyer’s lab will be one of five initial sites participating in this work.