AUSTIN, Texas — A group of scientists and engineers that includes researchers from The University of Texas at Austin has created a new class of materials that can absorb low energy light and transform it into higher energy light. The new material is composed of ultra-small silicon nanoparticles and organic molecules closely related to ones used in OLED TVs. This new composite efficiently moves electrons between its organic and inorganic components, with applications for more efficient solar panels, more accurate medical imaging and better night vision goggles.
The material is described in a new paper in Nature Chemistry.
“This process gives us a whole new way of designing materials,” said Sean Roberts, an associate professor of chemistry at UT Austin. “It allows us to take two extremely different substances, silicon and organic molecules, and bond them strongly enough to create not just a mixture, but an entirely new hybrid material with properties that are completely distinct from each of the two components.”
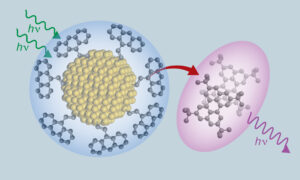
Composites are composed of two or more components that adopt unique properties when combined. For example, composites of carbon fibers and resins find use as lightweight materials for airplane wings, racing cars and many sporting products. In the paper co-authored by Roberts, the inorganic and organic components are combined to show unique interaction with light.
Among those properties are the ability to turn long-wavelength photons — the type found in red light, which tends to travel well though tissue, fog and liquids — into short-wavelength blue or ultraviolet photons, which are the type that usually make sensors work or produce a wide range of chemical reactions. This means the material could prove useful in new technologies as diverse as bioimaging, light-based 3D printing and light sensors that can be used to help self-driving cars travel through fog.
“This concept may be able to create systems that can see in near infrared,” Roberts said. “That can be useful for autonomous vehicles, sensors and night vision systems.”
Taking low energy light and making it higher energy also can potentially help to boost the efficiency of solar cells by allowing them to capture near-infrared light that would normally pass through them. When the technology is optimized, capturing low energy light could reduce the size of solar panels by 30%.
Members of the research team, which includes scientists from the University of California Riverside, University of Colorado Boulder and University of Utah, have been working on light conversion of this type for several years. In a previous paper, they described successfully connecting anthracene, an organic molecule that can emit blue light, with silicon, a material used in solar panels and in many semiconductors. Seeking to amplify the interaction between these materials, the team developed a new method for forging electrically conductive bridges between anthracene and silicon nanocrystals. The resulting strong chemical bond increases the speed with which the two molecules can exchange energy, almost doubling the efficiency in converting lower energy light to higher energy light, compared with the team’s previous breakthrough.
The research was funded by the National Science Foundation, The Welch Foundation, the W.M. Keck Foundation, and the Air Force Office of Scientific Research.
Kefu Wang and Ming Lee Tang of University of Utah, R. Peyton Cline and Joel D. Eaves of University of Colorado Boulder, Joseph Schwan and Lorenzo Mangolini of University of California Riverside and Jacob M. Strain of UT Austin also contributed extensively to the research.